Evolution of Complex Phenotypes and Regulatory Networks
GRNs provide a window into ways evolution can work on variation in ways that go far beyond selection among point mutations. Free IPAK-EDU Lecture accessible here!
Imagine the intricate control network in charge of the ballet of cells, molecules, and processes that shape the diversity of life on Earth. From the vibrant petals of a flower to the sophisticated structures of the human brain, this complexity is orchestrated by the finely tuned interactions within gene regulatory networks (GRNs). These networks, comprising genes, proteins, and other molecular components, control gene expression in a highly coordinated manner, enabling organisms to develop, adapt, and thrive in their environments.
(Free lecture here and below!)
Gene regulatory networks are essential for the biological processes that govern life. They are the systems of interacting components—genes, transcription factors, regulatory elements, and small molecules—that manage the levels at which genes are expressed in a cell. These networks are more than static blueprints; they are also dynamic systems that respond to both internal cues and external environmental changes. And, like blueprints that change patterns on the page as you’re building a building from them - GRNs themselves evolve. This responsiveness is crucial for maintaining cellular and metabolic homeostasis as environmental conditions change, guiding development as key morphological changes occur over time, and enabling physiological adaptations in response to selection pressures.
The journey from a single fertilized egg to a fully formed organism involves intricate orchestration of regulatory signals, each playing its part at precisely the right moment. This orchestration is the result of millions of years of evolution, where gene regulatory networks have become increasingly sophisticated. They have evolved from simple beginnings to complex, multi-layered systems capable of fine-tuning gene expression in response to myriad stimuli. This evolution is driven by mechanisms such as gene duplication, mutation, and natural selection, via which organisms can develop new traits and adapt to changing environments.
The concept of gene regulation has deep roots in scientific history, with early insights provided by the pioneering work on the lac operon in Escherichia coli by François Jacob and Jacques Monod in the 1960s. Their groundbreaking discovery illustrated how genes could be turned on or off in response to the presence or absence of specific molecules, such as lactose. This was one of the first explicit models of a gene regulatory network, showing how genes, promoters, operators, and repressors interact in a coordinated fashion to control gene expression.
Fig. 1 from Albergante et al. “The E. coli GRN derived from Salgado et al. (2012) using two evidence codes. Genes that are reported to regulate transcriptionally at least one other gene, that is transcription factors (TFs), are represented as red circles; the other genes are represented by blue circles. Arrows indicate a transcriptional interaction from the TF to the target gene. The arrows are colour-coded according to the number of genes regulated by the source TF. Note the logarithmic scale in the colour coding.”
As we explore the evolution of gene regulatory networks, it becomes clear that their complexity is not just a result of the number of components they contain but also the intricate ways they interact. These interactions form the basis of the cellular processes that lead to the development of complex phenotypes—distinct, observable characteristics such as the structure of the eye, the vertebrate limb, and even the sophisticated cognitive functions of the human brain.
Understanding the evolution of these networks has profound implications for modern science and medicine. By unraveling the intricacies of GRNs, scientists can develop new strategies for treating diseases, creating synthetic biological systems, and understanding the fundamental processes that drive life on Earth.
In this in-depth article, we will explore the evolution of gene regulatory networks in detail, examining the mechanisms that drive their complexity, the historical insights that have shaped our understanding, and the specific examples that illustrate their role in the evolution of complex phenotypes. This journey through the evolution of gene regulation and the regulation of entire regulatory programs promises to illuminate the delicate balance of structure and function that defines living organisms, providing a deeper appreciation for the evolutionary tapestry that underpins the diversity of life.
For those eager to explore these topics further, our course Principles of Evolution at IPAK-EDU offers a comprehensive exploration of evolutionary biology, bridging theoretical constructs with empirical data to foster a holistic understanding of evolution. Through this program, we equip learners with the knowledge and tools needed to explore all aspects of the evolution of ideas and knowledge in evolutionary biology.
Gene Regulatory Networks: Blueprints of Life
Gene regulatory networks (GRNs) are the intricate systems of interacting components that govern gene expression within a cell. At their core, these networks are composed of genes, transcription factors, and regulatory elements that work in concert to control the flow of genetic information. Understanding the structure and function of GRNs is crucial for comprehending how cells develop, function, and respond to their environment.
Building on the analogy of an orchestra, where each instrument (gene) must play its part at the right time and in harmony with others to produce a symphony (the phenotype). The conductors of this orchestra are the transcription factors—proteins that bind to specific DNA sequences and regulate the transcription of genetic information from DNA to messenger RNA. These transcription factors can activate or repress the expression of target genes, ensuring that the right genes are expressed at just the right levels at just the right times.
GRNs include genes, transcription factors, regulatory elements (promoters, enhancers, and silencers), and non-coding RNAs. The interaction among these components forms complex networks that dictate how genes are expressed in different tissues and under various conditions. This regulatory framework is essential for maintaining cellular homeostasis, driving development, and thus organisms adapt to environmental changes.
Gene regulatory networks are not static; they evolve through processes such as gene duplication, mutation, and natural selection. These evolutionary changes can lead to the emergence of new regulatory pathways and the fine-tuning of existing ones, enabling organisms to develop new traits and adapt to new environments.
One of the most profound examples of a GRN is the lac operon in Escherichia coli. Discovered by François Jacob and Jacques Monod, the lac operon is a cluster of genes involved in lactose metabolism. This operon includes genes encoding proteins that transport and metabolize lactose, as well as regulatory sequences that control the expression of these genes. The lac operon is regulated by the presence or absence of lactose and glucose, demonstrating how GRNs can integrate environmental signals to control gene expression dynamically.
Transcription factors are crucial in GRNs because they can act as master regulators, controlling the expression of multiple genes and thus orchestrating complex developmental programs. For example, the transcription factor p53 regulates the expression of genes involved in cell cycle arrest, apoptosis, and DNA repair, playing a critical role in preventing cancer.
The regulatory elements within GRNs are equally important. Promoters and enhancers can be considered switches and dials that control gene expression. Enhancers, in particular, can have a significant impact on the evolution of gene expression patterns. Changes in enhancer sequences can lead to differences in when, where, and how much a gene is expressed, contributing to phenotypic diversity and adaptation.
Understanding GRNs also has practical implications for modern science and medicine. Insights into GRNs can lead to advances in biotechnology, such as the development of improved biological systems for commercial processes and gene therapies. For instance, by manipulating GRNs, scientists can tweak networks that perform specific functions, such as producing biofuels or therapeutic proteins.
Moreover, studying GRNs can shed light on the molecular basis of diseases. Many diseases, including cancer, are caused by disruptions in gene regulation. By understanding how GRNs function and how they can go awry, researchers can develop targeted therapies to correct these regulatory defects.
The Evolution of Gene Regulatory Networks
Gene regulatory networks (GRNs) are dynamic entities that have evolved over millions of years, a period of time over which organisms have developed complex phenotypes and adapt to diverse environments. This evolution is driven by the interplay of several fundamental mechanisms, including gene duplication, mutation, and natural selection. Understanding these processes is crucial for unraveling the complexity of life and the adaptability of organisms.
One of the primary mechanisms driving the evolution of GRNs is gene duplication. When a gene is duplicated, it creates a copy that can acquire new functions or regulatory elements without affecting the original gene's function. This process can lead to the expansion and diversification of gene families, providing raw material for evolutionary innovation. For example, the duplication of zinc finger transcription factors has led to a significant increase in regulatory complexity in humans and other primates. These transcription factors, characterized by their zinc finger motifs, bind to DNA and regulate gene expression, playing critical roles in various biological processes.
Gene duplication does not act in isolation; it is often accompanied by other types of mutations that further diversify the functions of duplicated genes. These mutations can occur in the coding regions, altering the protein's structure and function, or in the regulatory regions, changing the gene's expression patterns. Over time, these changes can lead to the emergence of new regulatory pathways and the fine-tuning of existing ones, enhancing an organism's ability to adapt to its environment.
Regulatory network rewiring is another critical aspect of GRN evolution. This process involves changes in the interactions and regulatory mechanisms within the network, leading to new patterns of gene expression. For instance, in cichlid fish, evolutionary changes in GRNs driven by alterations in cis-regulatory modules have led to the development of distinct phenotypic traits. These changes underscore the importance of regulatory rewiring in adaptive evolution, enabling cichlids to thrive in diverse ecological niches.
Cis-regulatory modules (CRMs) are segments of DNA that regulate the expression of nearby genes by acting as binding sites for transcription factors, which can either activate or repress gene activity. They function like control panels, determining when, where, and how much a gene is expressed, essential for proper development and adaptation.
Cis-regulatory modules play a vital role in the evolution of GRNs by controlling the expression of genes in a context-dependent manner. Changes in CRMs can significantly impact the timing, location, and level of gene expression, leading to phenotypic diversity. For example, research has shown that modifications in CRMs contribute to the evolution of animal morphology by altering the functional organization of GRNs that control body plan development. In cardiac cell fate determination, specific changes in cis-regulatory elements define cell lineage and reflect evolutionary history, highlighting how regulatory modules can lead to the development of new cell types and functions.
Epigenetic modifications, such as DNA methylation, add another layer of complexity to gene expression regulation. Methylation can modulate gene expression without altering the DNA sequence, playing a critical role in development, physiology, and adaptation. Studies on the human brain have shown that differential methylation in regulatory regions contributes to unique gene expression profiles associated with brain development and function. These epigenetic changes are believed to play a crucial role in the cognitive and neurological differences between humans and other primates.
In another example, research on high-altitude adaptation in Tibetan populations has revealed that DNA methylation changes in the EPAS1 gene are linked to efficient oxygen transport and utilization, offering a survival advantage in hypoxic conditions. The EPAS1 gene, which plays a critical role in the body's response to low oxygen levels, shows different methylation patterns in Tibetans compared to lowland populations, enabling better adaptation to their environment (LINK).
Long-term Evolution of GRNs
Understanding the long-term evolution of gene regulatory networks (GRNs) provides profound insights into how complex traits and adaptations arise over extended periods. Long-term evolution experiments, such as those conducted with Escherichia coli and other model organisms, have demonstrated how GRNs can evolve to become more efficient at generating beneficial mutations, ultimately enhancing the adaptability and resilience of organisms.
Lenski's Long-Term Evolution Experiment with E. coli
One of the most remarkable studies in long-term evolution is Richard Lenski's Long-Term Evolution Experiment (LTEE), which began in 1988 and continues to this day. This experiment involves propagating 12 populations of E. coli under controlled conditions, allowing researchers to observe evolutionary changes over tens of thousands of generations. The LTEE has provided invaluable data on the dynamics of genetic and phenotypic evolution.
One of the key findings from the LTEE is the repeated evolution of beneficial mutations in specific genes, demonstrating the predictability of adaptive evolution. For instance, E. coli populations have repeatedly evolved mutations in genes involved in central metabolic pathways, such as the citrate synthase gene (citT). As result, they can utilize citrate as a carbon source under aerobic conditions. This adaptation, which occurred in one of the populations after about 31,000 generations, highlights how GRNs can be rewired to exploit new ecological niches.
Increased “Evolvability” through Network Modifications
The LTEE also sheds light on how GRNs can evolve to increase their “evolvability”—the capacity to generate adaptive genetic diversity. Over thousands of generations, E. coli populations have shown an increased ability to adapt to new environmental challenges. This increased evolvability is thought by some evolutionary biologists to be driven by the accumulation of mutations that enhance the flexibility and robustness of GRNs.
There is some evidence that GRN complexity may be optimized by natural selection to avoid being so overly complex and over-rigid that lineages avoid canalization: over-specialization to a narrow niche potential. Many classical evolutionary biologists eschew the concept of “Evolvability” because natural selection can only work on the variation that exists and that it can “see”; the idea that evolution might anticipate future circumstances required a “reading in” of the evolutionary process.
However, feature optimization is a well-known outcome of selection on past variation. A recent study explored the evolution of the lack of constraints via the restriction of the complexity and rigidity of GRNs.
Buffered Qualitative Stability (BQS) and Its Implications
Buffered Qualitative Stability (BQS) is a theoretical framework developed to explain or describe how gene regulatory networks (GRNs) maintain their robustness and evolvability. BQS posits that the organization of GRNs helps them to remain stable despite environmental and evolutionary changes. This stability is achieved by ensuring that the network's qualitative responses are maintained even when quantitative parameters, such as protein concentrations, fluctuate.
Key Concepts of BQS
1. Qualitative Stability: The network's ability to maintain stable gene expression patterns in response to perturbations.
2. Buffered Stability: The network's resilience to structural changes, such as the addition of new regulatory links.
Evidence and Predictions
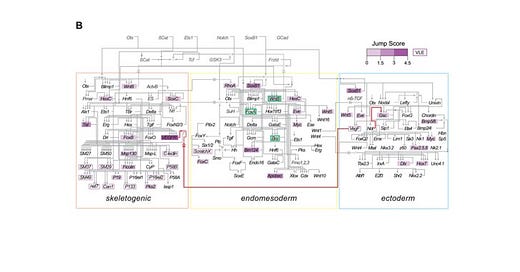
Albergante et al. demonstrated that BQS can predict several properties of GRNs. Remarkably, GRNs are free from long feedback loops involving more than three genes, which can lead to instability. GRNs are also known to have a high proportion of transcription factors (TFs) that are not regulated by other TFs, reducing the risk of unstable loops. GRNs are significantly more stable than randomly generated networks with similar size and connectivity.
The study found that the GRN of a human leukemia cell line (K562) deviates from BQS predictions, suggesting that the loss of BQS contributes to the phenotypic plasticity of cancer cells. This insight can help identify potential targets for cancer therapy.
Understanding BQS provides insights into how GRNs evolve to maintain stability and adaptability, helping explain the robustness of living organisms to genetic and environmental changes.
Cis-regulatory Module (CRM) Evolution
The evolution of cis-regulatory modules (CRMs) is another crucial aspect of long-term GRN evolution. CRMs are regions of DNA that contain binding sites for transcription factors, regulating the expression of associated genes. Changes in CRMs can significantly alter gene expression patterns, leading to phenotypic diversity and adaptation. For example, research on cichlid fish has shown that evolutionary changes in CRMs contribute to the development of distinct phenotypic traits, such as variations in jaw morphology, which are crucial for exploiting different ecological niches (LINK).
Evolution of Developmental Pathways
Long-term evolution also impacts developmental pathways regulated by GRNs. For instance, the evolution of limb development in vertebrates illustrates how GRNs can be modified over time to produce diverse morphological outcomes. Despite the vast differences in limb structures across species—from the wings of birds to the fins of fish—the underlying genetic networks controlling limb development are highly conserved. Changes in the timing, location, and intensity of gene expression within these networks, driven by modifications in CRMs, rendering the evolution of a wide array of limb forms suited to different environments (PLOS).
Epigenetic Modifications and Adaptation
Epigenetic modifications, such as DNA methylation, also play a significant role in the long-term evolution of GRNs. These modifications can alter gene expression patterns without changing the underlying DNA sequence, providing a rapid and reversible means of adaptation. For example, populations of Tibetan highlanders exhibit unique DNA methylation patterns in the EPAS1 gene, which enhance oxygen transport and utilization, offering a survival advantage in hypoxic conditions. Such epigenetic changes are crucial for quick adaptation to environmental challenges, complementing genetic mutations in driving evolutionary change (PLOS).
Future Directions in GRN Evolution Research
The study of long-term GRN evolution continues to evolve with advancements in genomic technologies and computational modeling. Future research aims to integrate multi-omic data, including genomics, transcriptomics, and epigenomics, to build comprehensive models of GRN evolution. These models will provide deeper insights into how genetic and epigenetic factors interact to shape the evolution of complex traits.
Understanding the long-term evolution of GRNs not only enriches our knowledge of evolutionary biology but also has practical implications for fields such as medicine and biotechnology. By unraveling the mechanisms underlying GRN evolution, scientists can develop novel strategies for disease treatment, genetic engineering, and the design of synthetic biological systems.
In summary, the long-term evolution of gene regulatory networks is a multifaceted process involving gene duplication, CRM evolution, regulatory network rewiring, and epigenetic modifications. These mechanisms collectively enhance the adaptability and complexity of organisms, driving the evolution of diverse phenotypes and enabling life to thrive in a diversity of environments.
Key Features of GRNs
Gene regulatory networks (GRNs) are characterized by several key features that enable them to regulate gene expression effectively and adapt to changing conditions. These features include feedback and feedforward loops, modularity, and the presence of network motifs. Understanding these structural and functional properties is crucial for deciphering the complexity of GRNs and their role in evolutionary biology.
Feedback and Feedforward Loops
One of the fundamental aspects of GRNs is the presence of feedback and feedforward loops. These loops are essential for regulating the stability and dynamics of gene expression.
Negative Feedback Loops: These loops help maintain gene expression within specific limits, stabilizing cellular functions. For example, in the lac operon of E. coli, the presence of lactose inhibits the repressor protein, resulting in the expression of genes involved in lactose metabolism. Once lactose is metabolized, the repressor rebinds, shutting down the operon. This type of feedback ensures that gene expression is tightly regulated in response to environmental changes (Oxford Academic).
Positive Feedback Loops: These loops amplify gene expression, creating bistable states where a gene is either fully on or off. This can lead to the establishment of distinct cell states, crucial for processes like cell differentiation. For instance, the activation of certain transcription factors during development can reinforce their own expression, leading to stable cell fate decisions (Oxford Academic) (PLOS).
Feedforward Loops
Feedforward loops involve a regulatory sequence where one gene regulates a second gene and jointly regulates a third gene. These structures are essential for creating time delays in gene expression, filtering noise, and ensuring robust responses to stimuli. For example, in the regulation of the developmental gene network in Drosophila, feedforward loops help to stabilize the expression patterns of key developmental genes, ensuring precise spatial and temporal control (LINK).
Modularity
Modularity refers to the organization of GRNs into semi-independent clusters or modules that perform specific functions. This structural property is crucial for the robustness and evolvability of GRNs.
Robustness: Modularity facilitates the independent functioning of individual modules. This minimizes the impact of mutations or environmental changes on the overall network. This enhances the network's ability to maintain stable gene expression patterns despite perturbations.
Material for Future Innovations: Modularity enables the reorganization or co-option of existing modules for new functions, facilitating evolutionary innovations. For example, the development of vertebrate limbs involves the modular activation of gene clusters that regulate different aspects of limb formation. Changes in these modules can lead to the evolution of diverse limb structures without disrupting the core regulatory framework (LINK)
Examples of Modularity
Vertebrate Limb Development: The genetic network controlling limb development in vertebrates is highly modular, with distinct modules regulating processes such as limb outgrowth and digit formation. Changes in the regulatory elements of these modules have led to the evolution of various limb types, such as wings in birds and fins in fish (LINK)
Flower Development in Plants: In plants, the development of flowers is regulated by modular gene networks. The ABC model of flower development describes how different combinations of gene modules specify the identity of floral organs, providing material for the diversity of flower forms observed across plant species (PLOS).
Network Motifs
Network motifs are recurring, significant patterns of interconnections within GRNs. These motifs represent the basic building blocks of the network and are thought to play key roles in regulatory functions.
Feedforward Loops: As mentioned earlier, feedforward loops are crucial for creating time delays and ensuring robust gene expression responses.
Single-Input Modules: In these motifs, a single transcription factor regulates multiple target genes. Coordinated regulation of functionally related genes, ensuring that they are expressed in a synchronized manner, can result (Scinapse) (BMC) (ScienceOfBiogenetics).
Dense Overlapping Regulons: These motifs involve a dense network of transcription factors and target genes, leading to complex regulatory interactions. This structure is often seen in networks regulating critical cellular processes, such as the stress response and cell cycle control (Alon, 2007).
Functional Consequences of Network Motifs
Network motifs contribute to the functional properties of GRNs by providing mechanisms for integrating multiple signals, creating robust and adaptable gene expression patterns, and enabling complex regulatory behaviors. For instance, in the development of the fruit fly Drosophila melanogaster, network motifs ensure the precise spatial and temporal regulation of genes required for segment formation, leading to the proper development of the individual. So, along with HOX genes, variations on a theme can be severely constrained.
As general overview, the evolution of gene regulatory networks (GRNs) likely began with simple regulatory mechanisms and progressively became more sophisticated. Initially, basic forms of gene regulation involved straightforward promoter regions directly controlling gene expression. These early systems were then augmented by the duplication of transcription factors, which, through subsequent mutations, acquired new regulatory functions. This process often resulted in the loss of function of promoters in duplicated genes, necessitating the evolution of alternative regulatory elements like enhancers and silencers to maintain gene expression.
As GRNs evolved, regulatory motifs such as feedback and feedforward loops emerged, resulting in more precise control of gene activity. Additionally, alternative splicing mechanisms were integrated, further increasing the diversity of gene products and enhancing the complexity of regulatory networks. Over time, these networks became modular, with distinct modules capable of independent evolution and adaptation. The final layers of sophistication involved epigenetic modifications, like DNA methylation, which provided an additional level of gene expression control, resulting in for fine-tuning in response to environmental and developmental cues.
Conclusion
The key features of GRNs, including feedback and feedforward loops, modularity, and network motifs, are essential for understanding how these networks regulate gene expression and drive evolutionary change. These structural and functional properties enable GRNs to maintain stability, adapt to environmental changes, and evolve new regulatory functions. By studying these features, scientists can gain deeper insights into the mechanisms underlying the evolution of complex phenotypes and the adaptability of life on Earth.
Practical Implications of GRN Evolution
Understanding gene regulatory networks (GRNs) and their evolution has profound implications for various fields, including medicine, biotechnology, and synthetic biology. By grasping the mechanisms that drive GRN evolution, scientists can develop innovative strategies to manipulate these networks for practical applications.
Advances in Medicine
Understanding GRNs is crucial for unraveling the molecular basis of diseases. Many diseases, including cancer, diabetes, and neurological disorders, are caused by disruptions in gene regulation. For instance, malfunctioning regulatory networks can lead to uncontrolled cell proliferation in cancer. By mapping the GRNs involved in such diseases, researchers can identify critical regulatory nodes and develop targeted therapies to correct these defects.
Cancer Research and Treatment
Cancer is often driven by mutations that alter the expression and function of genes within GRNs. For example, mutations in the p53 gene, a crucial tumor suppressor, disrupt its regulatory network, leading to unchecked cell growth. By understanding the network interactions of p53 and other oncogenes, researchers can design drugs that restore normal regulatory functions or selectively target cancer cells. Recent advancements in genome editing technologies, such as CRISPR-Cas9, have enabled precise modifications of GRNs, offering new avenues for cancer therapy.
Genetic and Epigenetic Therapies
The evolution of GRNs also sheds light on the potential for genetic and epigenetic therapies. Diseases caused by aberrant gene expression, such as certain inherited disorders, can be treated by correcting the underlying regulatory defects. For instance, sickle cell anemia, caused by a single mutation in the hemoglobin gene, could be addressed by editing the regulatory elements to restore normal gene expression. Additionally, epigenetic modifications, such as DNA methylation, play a significant role in gene regulation and can be targeted to treat diseases like Fragile X syndrome and Rett syndrome.
Biotechnology Applications
In biotechnology, the principles of GRN evolution are applied to engineer organisms with desired traits. By manipulating GRNs, scientists can enhance the production of biofuels, pharmaceuticals, and other valuable compounds. For example, by engineering the regulatory networks of yeast, researchers have developed strains that efficiently produce bioethanol from agricultural waste. This approach not only increases the yield but also makes the process more sustainable and cost-effective.
Synthetic Biology
Synthetic biology involves designing and constructing new biological parts, devices, and systems by leveraging the principles of GRNs. By understanding how natural GRNs evolve, scientists can create synthetic networks that perform specific functions. For example, synthetic biologists have designed genetic circuits that control the timing and intensity of gene expression, leading to precise control over cellular behavior. These synthetic networks can be used in a variety of applications, from biosensors that detect environmental pollutants to programmable cells that produce therapeutic proteins in response to disease signals.
Environmental Adaptation and Conservation
The study of GRNs also has implications for environmental adaptation and conservation. By understanding how organisms adapt to changing environments through the evolution of GRNs, conservation biologists can develop strategies to protect endangered species. For instance, by identifying the GRNs involved in stress responses, researchers can predict how species might adapt to environmental change and challenges and develop conservation plans that enhance their resilience.
Agricultural Improvements
In agriculture, manipulating GRNs can lead to the development of crops with improved traits, such as increased yield, pest resistance, and drought tolerance. For example, by modifying the regulatory networks that control flowering time in plants, scientists can create crop varieties that bloom at optimal times, increasing productivity. Additionally, understanding the GRNs that regulate root development can lead to crops with enhanced nutrient uptake and better performance in poor soils.
Comprehensive Conclusion
The study of gene regulatory networks (GRNs) and their evolution provides profound insights into life's complexity and adaptability. These intricate networks of genes, transcription factors, and regulatory elements orchestrate the expression of genes in precise spatial and temporal patterns, enabling organisms to develop, adapt, and thrive in diverse environments. Through mechanisms such as gene duplication, regulatory network rewiring, and changes in cis-regulatory modules, GRNs evolve to generate the phenotypic diversity that characterizes the living world.
We explored the critical roles of feedback and feedforward loops, modularity, and network motifs in maintaining the stability and robustness of GRNs. In a way sometimes perhaps misinterpreted as prescient evolution, these features not only enhance the adaptability of organisms but also facilitate evolutionary innovations. The examples of Hox genes, methylation patterns, and long-term evolution experiments, such as Richard Lenski's work with E. coli, underscore how changes in GRNs drive the evolution of complex phenotypes and enable organisms to exploit new ecological niches.
The practical implications of understanding GRN evolution are vast, impacting fields such as medicine, biotechnology, synthetic biology, environmental conservation, and agriculture. By unraveling the complexities of GRNs, scientists can develop targeted therapies for diseases, engineer organisms with desirable traits, and design synthetic biological systems with specific functions. This knowledge paves the way for innovative solutions to some of the most pressing challenges facing humanity, from improving health and sustainability to enhancing the resilience of ecosystems.
In summary, the evolution of gene regulatory networks is a multifaceted and dynamic process that underpins the diversity of life. Continued research in this area promises to unlock new possibilities for understanding and manipulating the genetic basis of development, adaptation, and evolution. For those eager to explore these topics further, our course at IPAK-EDU offers a comprehensive exploration of evolutionary biology, bridging theoretical constructs with empirical data to foster a holistic understanding of evolution.
The journey through the intricate web of gene regulation is a gateway to discovering the fundamental principles that govern life and drive the evolutionary processes that have shaped our living world.
References
Jacob, F., & Monod, J. (1961). Genetic regulatory mechanisms in the synthesis of proteins. Journal of Molecular Biology, 3(3), 318-356. https://doi.org/10.1016/S0022-2836(61)80072-7
Wang, X., Hou, J., Quedenau, C., & Chen, W. (2016). Pervasive isoform-specific translational regulation via alternative transcription start sites in mammals. Molecular Systems Biology, 12(2), 875. https://doi.org/10.15252/msb.20156768
Wray, G. A. (2007). The evolutionary significance of cis-regulatory mutations. Nature Reviews Genetics, 8(3), 206-216. https://doi.org/10.1038/nrg2063
Stern, D. L., & Orgogozo, V. (2008). The loci of evolution: How predictable is genetic evolution? Evolution, 62(9), 2155-2177. https://doi.org/10.1111/j.1558-5646.2008.00450.x
Reik, W., & Walter, J. (2001). Genomic imprinting: Parental influence on the genome. Nature Reviews Genetics, 2(1), 21-32. https://doi.org/10.1038/35047554
Lenski, R. E., & Travisano, M. (1994). Dynamics of adaptation and diversification: A 10,000-generation experiment with bacterial populations. Proceedings of the National Academy of Sciences, 91(15), 6808-6814. https://doi.org/10.1073/pnas.91.15.6808
Lenski, Richard (February 15, 2023). "Revisiting the Design of the Long-Term Evolution Experiment with Escherichia coli". Journal of Molecular Evolution. 91 (1): 241–253.
Henning, F., Machado-Schiaffino, G., Baumgarten, L., Meyer, A. (2020). Evolution of regulatory networks associated with traits under selection in cichlids. Genome Biology, 21, 117. https://doi.org/10.1186/s13059-020-02208-8
Ilya Ruvinsky, Jeremy J. Gibson-Brown; Genetic and developmental bases of serial homology in vertebrate limb evolution. Development 15 December 2000; 127 (24): 5233–5244. doi: https://doi.org/10.1242/dev.127.24.5233
Liao, B.-Y., & Zhang, J. (2008). Null mutations in human and mouse orthologs frequently result in different phenotypes. Proceedings of the National Academy of Sciences, 105(19), 6987-6992. https://doi.org/10.1073/pnas.0800387105
Toth, A. L., & Robinson, G. E. (2007). Evo-devo and the evolution of social behavior. Trends in Genetics, 23(7), 334-341. https://doi.org/10.1016/j.tig.2007.05.001
Weldemichael, T., Asemoloye, M. D., & Marchisio, M. A. (2022). Feedforward Loops: Evolutionary Conserved Network Motifs Redesigned for Synthetic Biology Applications. Applied Sciences, 12(16), 8292. https://doi.org/10.3390/app12168292
Albergante, L., Blow, J. J., & Newman, T. J. (2014). Buffered Qualitative Stability explains the robustness and evolvability of transcriptional networks. eLife, 3, e02863. https://doi.org/10.7554/eLife.02863